Higher and foundation tiers
Balancing chemical equations

If you think about what happens to the particles during a chemical reaction you
would probably realise that all the particles present in the reactants appear in the products.
All that really happens during a chemical reaction is that the particles rearrange themselves as they change from reactants into products.
This idea is
really quite simple but crucial in understanding what happens during chemical reactions.
The law of conservation of mass states that the total mass of the reactants must be equal to the total mass of the products; nothing appears or disappears
during chemical reactions.
All the particles that you start with you end up; they will probably be in a different form but they are all still there!
Balancing equations
When we balance symbolic equations all we are doing really is simply counting atoms, to make sure that all the atoms are accounted for
and none are lost or have gone missing. Technically it's not atoms that we are counting it's the number of moles of reactants and products in the chemical reaction; but then again moles are just a way of counting large numbers of atoms. The only way to master the skill of balancing equations is to practice it. Luckily there are few simple
rules which can make it easier for us.
Working out chemical formulae is another useful skill which is easy to master and will improve your confidence in your
chemistry work. However on this page do not worry about any of the formulae for any of the reactants or products just concentrate on balancing the equations; if
you would like more help on working out formula then simply click here.
Worked examples
Below are some worked examples on how to balance chemical equations. Go through the worked examples and then try to balance equations yourself by completing the practice questions. The only way to get good at balancing equations is to balance lots of them, it's often trial and error until you get more confident at balancing equations.
Example 1.
Magnesium ribbon burns in air with a brilliant white flash to form magnesium oxide. We can represent this reaction with word and symbolic equations as shown below:
Word equation:
magnesium + oxygen → magnesium oxide
Symbolic equation:
Hint: Remember that oxygen gas is a diatomic gas, its goes around in pairs!
Mg + O2 → MgO
Model equation:
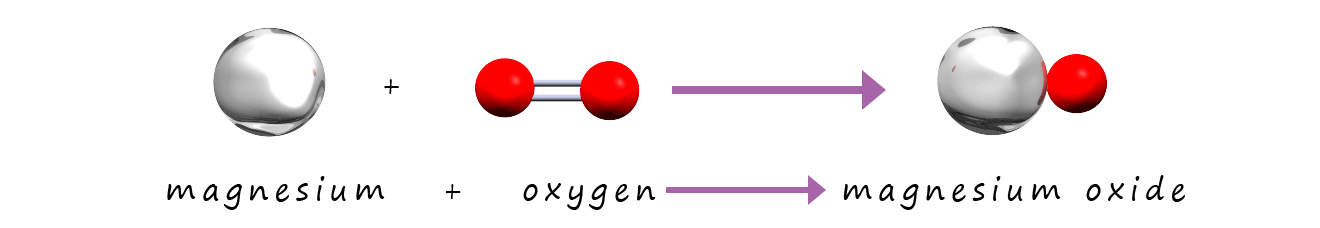 Now if you look carefully at the symbolic and the model equations you will see that they are not balanced. On the reactants side of the equation
there is 1 atom/mole of magnesium and 2 atoms/moles of oxygen. On the products side of the equation there is 1 atom/mole of magnesium and 1 atom/mole of oxygen.
We are missing 1 atom/mole of oxygen on the products side of the equation. Now the simple but
incorrect way to fix this is simply to
change the formula for magnesium oxide as shown below:
Now if you look carefully at the symbolic and the model equations you will see that they are not balanced. On the reactants side of the equation
there is 1 atom/mole of magnesium and 2 atoms/moles of oxygen. On the products side of the equation there is 1 atom/mole of magnesium and 1 atom/mole of oxygen.
We are missing 1 atom/mole of oxygen on the products side of the equation. Now the simple but
incorrect way to fix this is simply to
change the formula for magnesium oxide as shown below:
Mg + O2 → MgO2
However MgO2 is not the correct formula for magnesium oxide!
To balance equations the first rule is simple - DO NOT change any of the formula for the compounds you are given. You balance
equations by putting numbers in front of each of the reactants and products formulae until the number of atoms on each side of
the equation balances.
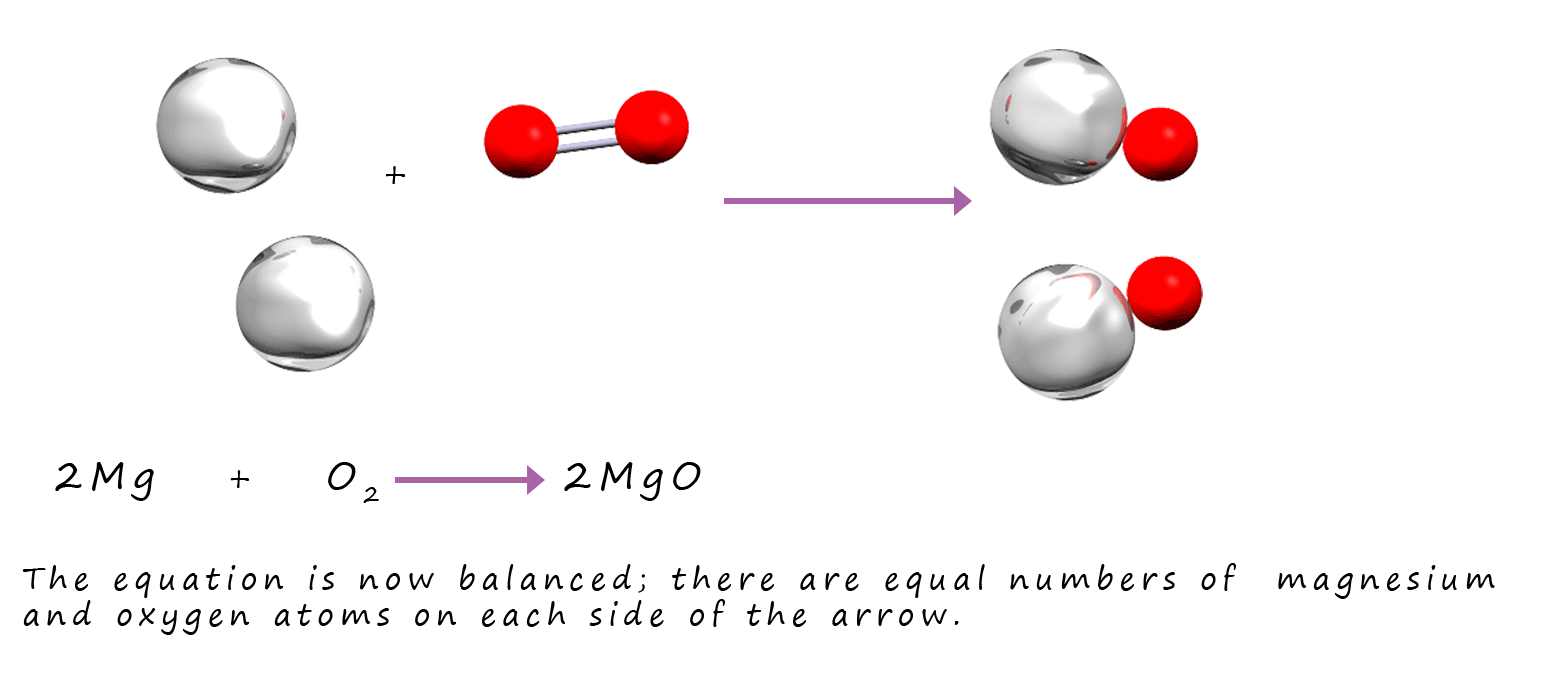
By inserting "2" into the equation above there are now equal numbers of atoms of each element on the reactants and products side of the equation.
Example 2.
Hydrogen burns in air to form hydrogen oxide or water. We can represent this reaction with word and symbolic equations:
Word equation:
hydrogen + oxygen → hydrogen oxide
Symbolic equation:
H2 + O2→ H2O
Model equation:
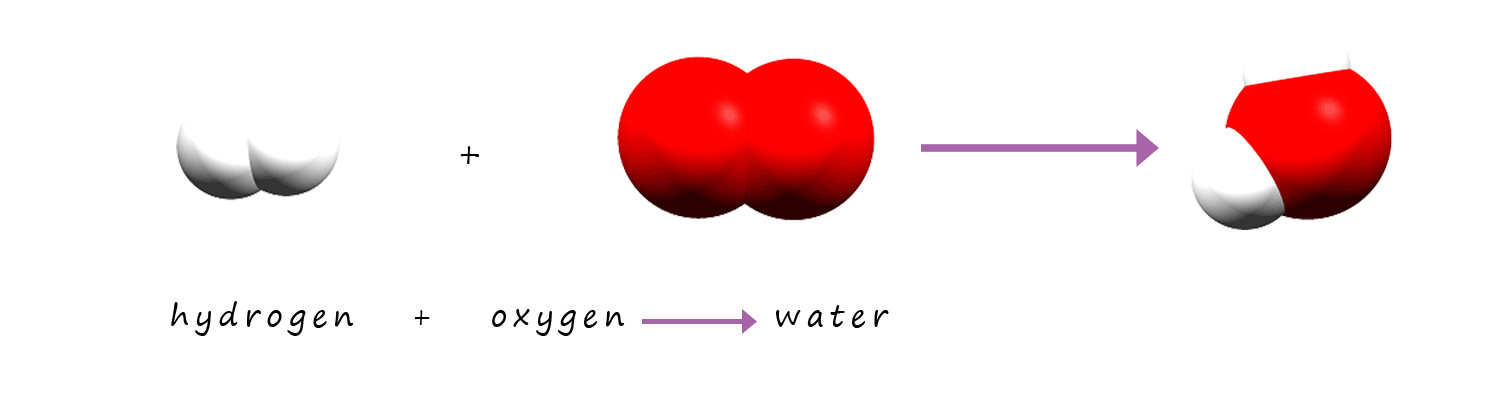 As in example 1 this equation is not balanced. There are 2 atoms of oxygen present on the reactants side of the equation and only 1
atom on the product side. So remember to balance the equation you can only put numbers in the front of each substance,
you cannot change any of the formula given.
As in example 1 this equation is not balanced. There are 2 atoms of oxygen present on the reactants side of the equation and only 1
atom on the product side. So remember to balance the equation you can only put numbers in the front of each substance,
you cannot change any of the formula given.
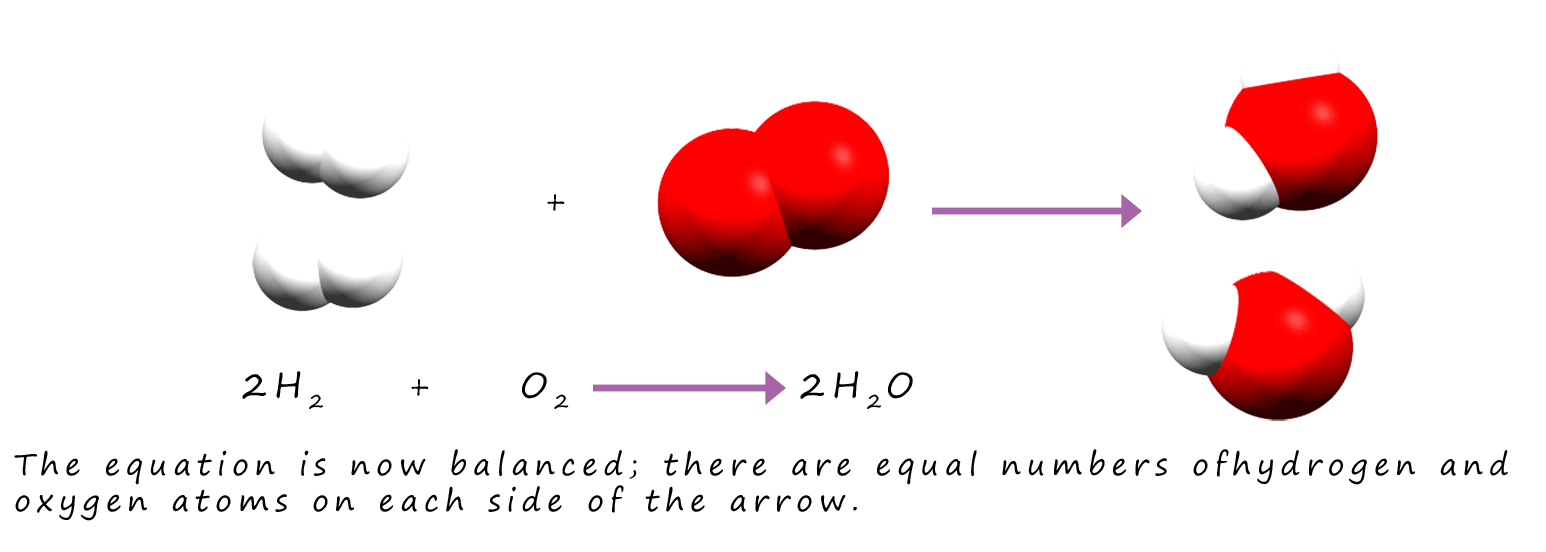 By inserting "2" into the equation above we now have 4 hydrogen atoms on the reactants side and 4 on the products side of the equation. There are 2 oxygen atoms on each
side of the equation so it is balanced.
By inserting "2" into the equation above we now have 4 hydrogen atoms on the reactants side and 4 on the products side of the equation. There are 2 oxygen atoms on each
side of the equation so it is balanced.
Example 3.
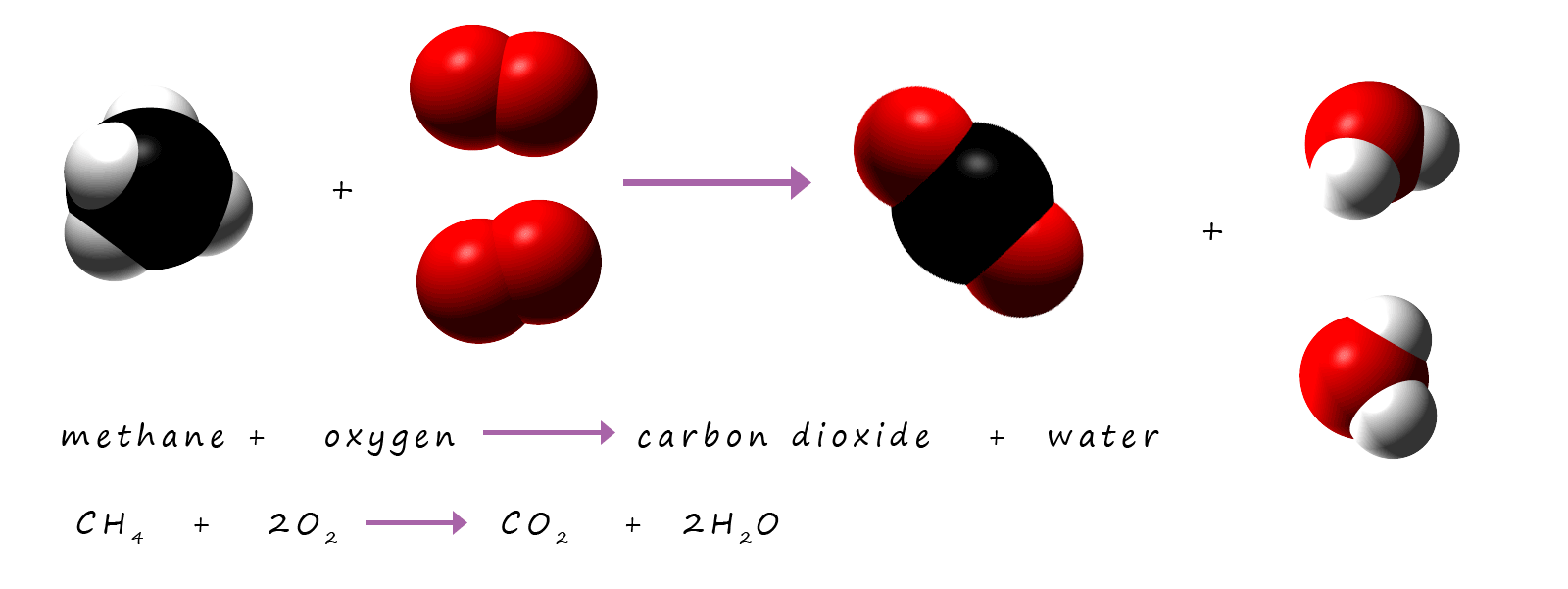
Methane is the gas used in Bunsen burners; it burns to form carbon dioxide and water vapour. We can represent this reaction with word and symbolic equations:
Word equation:
methane + oxygen → carbon dioxide + hydrogen oxide
Symbolic equation:
CH4 + O2 → CO2 + H2O
This equation is not balanced; there are not enough hydrogen atoms on the products side of the equation and too many oxygen atoms
on the products side of the equation. To make balancing equations which contain hydrogen and oxygen as well as other elements easier to balance as a general rule
balance the oxygen atoms last; the hydrogen atoms second last and any other elements first.
This equation can be balanced by:
- First check the carbon atoms on both sides of the equation, these balance as there is only one carbon atom on the reactants and products side of the equation.
Next
- Check the number of hydrogen atoms on both sides of the equation. On the reactants side there are 4 hydrogen atoms but only 2 on the product side of the equation, so to balance them place a "2" in front of the water on the products side of the equation. This gives 4 hydrogen atoms on both sides of the equation. This gives:
CH4 + O2 → CO2 + 2H2O
Finally check the number of atoms of oxygen atoms present on each side of the equation. Here there are 2 atoms of oxygen present on the reactant side of the equation but there are 4 atoms of oxygen on the products side of the equation, so to balance the oxygen atoms insert a "2" in front of the oxygen in the reactants side of the equation:
CH4 + 2O2 → CO2 + 2H2O
The equation is now balanced.
It is worth mentioning that really the only way to master balancing equations is to have a go yourself.
So please click the link below for the practice questions. You should also be aware that in the examples above we were trying to balance the
number of atoms of each element in the equations, however strictly speaking we should be talking about
balancing the number of moles of each substance
present.
Self-check
Practice your skills at balance equations by simply balancing the equations below. If no coefficient is needed in front of any reactant or product then enter a "1", also do not use fractions such as 1/2 to balance the equations, instead double all the quantities to balance the equations without using fractions.
Balance the equations below:
Enter whole-number coefficients (use 1 if no number is needed). Click Check, then Next.
Key points
- To balance equations do not change the formulae for any of the reactants or products but simply put numbers
in front of the formula for the reactant and products where necessary to balance the number of moles of each element on both sides of the equation.
- With equations containing lots of elements including hydrogen and oxygen it is often easier to balance these equations if
you balance the oxygen atoms last and the hydrogen atoms second last and the other elements present in any order.
Practice questions
Next


 Now if you look carefully at the symbolic and the model equations you will see that they are not balanced. On the reactants side of the equation
there is 1 atom/mole of magnesium and 2 atoms/moles of oxygen. On the products side of the equation there is 1 atom/mole of magnesium and 1 atom/mole of oxygen.
We are missing 1 atom/mole of oxygen on the products side of the equation. Now the simple but
incorrect way to fix this is simply to
change the formula for magnesium oxide as shown below:
Now if you look carefully at the symbolic and the model equations you will see that they are not balanced. On the reactants side of the equation
there is 1 atom/mole of magnesium and 2 atoms/moles of oxygen. On the products side of the equation there is 1 atom/mole of magnesium and 1 atom/mole of oxygen.
We are missing 1 atom/mole of oxygen on the products side of the equation. Now the simple but
incorrect way to fix this is simply to
change the formula for magnesium oxide as shown below:

 As in example 1 this equation is not balanced. There are 2 atoms of oxygen present on the reactants side of the equation and only 1
atom on the product side. So remember to balance the equation you can only put numbers in the front of each substance,
you cannot change any of the formula given.
As in example 1 this equation is not balanced. There are 2 atoms of oxygen present on the reactants side of the equation and only 1
atom on the product side. So remember to balance the equation you can only put numbers in the front of each substance,
you cannot change any of the formula given.
 By inserting "2" into the equation above we now have 4 hydrogen atoms on the reactants side and 4 on the products side of the equation. There are 2 oxygen atoms on each
side of the equation so it is balanced.
By inserting "2" into the equation above we now have 4 hydrogen atoms on the reactants side and 4 on the products side of the equation. There are 2 oxygen atoms on each
side of the equation so it is balanced. 